Products List
Benzyl Chloride
 |
Benzyl Chloride
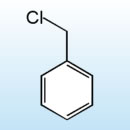 Benzyl chloride, or a-chlorotoluene, is an organic compound consisting of a phenyl group substituted with a chloromethyl group.
Benzyl chloride is used in organic synthesis for the introduction of the benzyl protecting group for alcohols (yielding the corresponding benzyl ether) and carboxylic acids (yielding the corresponding benzyl ester). It may be used in the synthesis of amphetamine-class drugs, and for this reason sales of benzyl chloride are monitored as a List II drug precursor chemical by the Drug Enforcement Administration in the United States of America.Benzyl chloride reacts with water in a hydrolysis reaction to form benzyl alcohol and hydrogen chloride. When the latter is dissolved in water, it forms hydrochloric acid.
Since benzyl chloride is quite volatile at room temperature, it can easily reach the mucous membranes where the hydrolysis takes place with production of hydrochloric acid. This explains why benzyl chloride is a lachrymator and has been used as a war gas. It is also very irritating to the skin.
Benzyl chloride also reacts readily with metallic magnesium to produce a Grignard Reagent.
A commonly used parent compound is benzyl bromide.
|
