Products List
Cinnamic Aldehyde
 |
Cinnamic Aldehyde
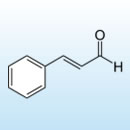 Cinnamic aldehyde or cinnamaldehyde (more precisely trans-cinnamaldehyde, the only naturally-occurring form) is the chemical compound that gives cinnamon its flavor and odor..
Cinnamaldehyde occurs naturally in the bark of cinnamon trees and other species of the genus Cinnamomum like camphor and cassia. These trees are the natural source of cinnamon, and the essential oil of cinnamon bark is about 90% cinnamaldehyde.
A yellow oily liquid more viscous than water, cinnamaldehyde smells strongly of cinnamon.
Concentrated cinnamaldehyde is a skin irritant, and the chemical is toxic in large doses, but no agencies suspect the compound is a carcinogen or poses a long-term health hazard.
Most cinnamaldehyde is excreted in urine as cinnamic acid, an oxidized form of cinnamaldehyde. Both aromatic and an aldehyde, cinnamaldehyde has a mono-substituted benzene ring. A conjugated double bond (alkene) makes geometry of the compound planar. Cinnamaldehyde is technically trans-cinnamaldehyde because the terminal carbonyl is on the opposite side of the aromatic ring over the rigid double bond.
Several methods of laboratory synthesis are now known, but cinnamaldehyde is most economically obtained from the steam distillation of the oil of cinnamon bark. The compound can be prepared from related compounds like cinnamyl alcohol, (the alcohol form of cinnamaldehyde), but the first synthesis from unrelated compounds was the aldol condensation of benzaldehyde and acetaldehyde.
|
